cellulose acetate
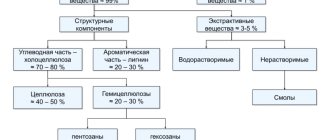
As a result of the interaction of cellulose with a mixture of acetic anhydride and acetic acid, cellulose acetate esters are formed - cellulose acetate. To obtain cellulose acetate, refined wood cellulose can be used, but the main raw material so far is cotton cellulose (linter). Cellulose acetate is used to produce silk acetate, cellon (non-flammable plastic), varnishes, film and other products. Cellulose dissolves in an ammonia cupric oxide solution to form a copper-ammonia complex compound, which is used to produce very fine copper-ammonium fiber. When cellulose interacts with water in the presence of catalysts, a hydrolysis reaction occurs and the simplest sugar is formed - glucose. Mineral acids are usually used as catalysts (acid hydrolysis).
hemicellulose
This concept unites a group of substances that are similar in chemical composition to cellulose, but differ from it in their ability to easily hydrolyze and dissolve in dilute alkalis. Hemicelluloses are mainly polysaccharides: pentosans and hexosans with five or six carbon atoms in the main unit. The degree of polymerization of hemicelluloses is much less than that of cellulose, i.e. the chains of molecules are shorter. The hydrolysis of hemicellulose polysaccharides produces simple sugars (monosaccharides); hexosans turn into hexoses, and pentosans turn into pentoses. Hemicelluloses are not typically obtained from wood as commercial products. However, in the chemical processing of wood they are widely used to obtain many valuable substances. For example, when wood is heated with 12% hydrochloric acid, almost all pentosans (93-96%) turn into simple sugars - pentoses - and after three molecules of water are separated from each monosaccharide molecule, furfural is formed - a product widely used in industry. In a growing tree, hexosans are storage substances, and pentosans perform a mechanical function.
lignin
In addition to carbohydrates (cellulose and hemicelluloses), the cell membrane contains an aromatic compound - lignin, which has a high carbon content. Cellulose contains 44.4% carbon, and lignin 60-66%. Lignin is less stable than cellulose and easily goes into solution when wood is treated with hot alkalis, aqueous solutions of sulfurous acid or its acid salts. This is the basis for the production of technical cellulose. Lignin is obtained in the form of waste during the cooking of sulfite and sulfate cellulose, and during the hydrolysis of wood. The lignin contained in black alkalis is mainly burned during regeneration.
Lignin is used as pulverized fuel, a substitute for tannins, in the production of fasteners, molding lands (in the foundry industry), plastics, artificial resins, for the production of activated carbon, vanillin, etc. However, the issue of the full qualified chemical use of lignin has not yet been resolved. Of the remaining organic substances contained in wood, resins and tannins have received the greatest industrial use.
Passport (certificate) for wood
Russian legislation does not require mandatory certification of lumber. But manufacturers planning to participate in tenders or export their products can apply for a voluntary certificate. It guarantees the quality and safety of products. The customer of the certificate for lumber can independently choose laboratory testing parameters.
In conclusion, you can watch an educational film that will once again tell you about the properties of wood:
resins
This group of substances is usually divided into water-insoluble resins (liquid and solid) and gums containing water-soluble gums. Among liquid resins, the most important is oleoresin, which is obtained from wood (sometimes from the bark) of coniferous species as a result of tapping. Tapping of pine and cedar is carried out as follows. In the fall, a vertical groove is made using special tools on a section of the trunk cleared of coarse bark, and with the onset of warm weather in the spring, strips of bark and wood directed at an angle of 30° to the groove are systematically removed and so-called undercuts are formed. The depth of the undercuts is usually 3-5 mm. The wound caused to a tree by tapping is called karra (Fig. 29).
From the cut resin passages, resin, under a pressure of 10-20 atm, flows into the shoeing and is directed along a groove to the receiver. After applying four to five refills, resin is removed from the conical receiver using a steel spatula. To increase the yield of resin, chemical stimulants (bleach or sulfuric acid) are used, which are used to treat the freshly exposed surface of the wood.
Tapping spruce is carried out by applying karra in the form of narrow longitudinal stripes. To obtain resin from larch, channels are drilled deep into the trunk until they meet large resin “pockets,” which often form in the lower part of the trunk. Larch resin is highly valued and is used in the paint and varnish industry for the production of the best types of varnishes and enamel paints. Fir resin is extracted from “blisters” that form in the bark. The resin from the punctured “blisters” is squeezed into portable receivers. Fir resin resembles Canada balsam in its properties and is used in optics, microscopic technology, etc.
The largest quantities of pine resin are obtained, which is a transparent resinous liquid with a characteristic pine odor. In air, the resin hardens and turns into a fragile whitish mass - barras. The pine resin obtained as a result of tapping contains approximately 75% rosin and 19% turpentine, the rest is water. Resin can be considered as a solution of solid resin acids (rosin) in liquid turpentine oil (turpentine). The processing of oleoresin is carried out at rosin-turpentine plants and consists of distilling off the volatile part, turpentine, with water vapor. The remaining non-volatile part is rosin.
Turpentine and rosin can be obtained by extraction processing of stump osmol - the core part of pine stumps, enriched with resin due to the rotting of low-resinous sapwood. Gasoline is most often used as a solvent. The resulting extract is distilled. The solvent and turpentine are distilled off, but the rosin remains. Extraction products are inferior in quality to turpentine and rosin obtained from resin. Turpentine is widely used as a solvent in the paint and varnish industry, for the production of synthetic camphor and other products. Camphor is used in large quantities as a plasticizer in the production of celluloid, varnishes and film.
The main consumer of rosin is the soap industry, where it is used to make laundry soap. Rosin glue is used in large quantities for gluing papers. Glycerol ether of rosin is added to nitro varnishes to add shine to the film. Rosin is used for the preparation of electrical insulating materials, in the production of synthetic rubber, etc. Larch gum is of great industrial importance. Gum is extracted from crushed wood with acidic water (acetic acid concentration 0.2%) at a temperature of 30°. After evaporation to a concentration of 60-70%, a commercial product is obtained. It is used in textile production for the production of paints, in the printing and paper industries, etc.
tannins or tannins
This concept unites all substances that have the properties of tanning raw leather, giving it resistance to rotting, elasticity, and the ability not to swell. The wood that is richest in tannins is oak kernel (6-11%) and chestnut (6-13%). The bark of oak, spruce, willow, larch and fir contains from 5 to 16% tannins. The growths on oak leaves - galls - contain from 35% to 75% tannins (one of the types of tannins). In bergenia leaves and roots, the tannin content is 15-25%.
Tynides are soluble in water and alcohol, have an astringent taste, when combined with iron salts they give a dark blue color, and are easily oxidized. Tannins are extracted with hot water from crushed wood and bark. The commercial product is either a liquid or dry extract, which is obtained after evaporating the solution in a vacuum apparatus and drying. Essential oils, lactoresins and dyes can also be obtained from woody plants.
Several ways to calculate the cubic capacity of rounded and cone-shaped logs
We will send the material by email
- 3 rooms
- 1 bathroom
- 93.8² Total area
- 5 rooms
- 2 bathrooms
- 186.2² Total area
- 5 rooms
- 2 bathrooms
- 144.2² Total area
- 4 rooms
- 2 bathrooms
- 128.3² Total area
- 4 rooms
- 2 bathrooms
- 144.5² Total area
- 1 room
- 2 bathrooms
- 315.5² Total area
- 8 rooms
- 2 bathrooms
- 228.9² Total area
- 7 rooms
- 2 bathrooms
- 179.5² Total area
- 4 rooms
- 2 bathrooms
- 157.5² Total area
- 6 rooms
- 3 bathrooms
- 167² Total area
- 6 rooms
- 2 bathrooms
- 131.7² Total area
- 4 rooms
- 2 bathrooms
- 151² Total area
- 5 rooms
- 2 bathrooms
- 184.7² Total area
- 4 rooms
- 2 bathrooms
- 121² Total area
- 5 rooms
- 2 bathrooms
- 155.5² Total area
- 5 rooms
- 2 bathrooms
- 237.2² Total area
- 5 rooms
- 3 bathrooms
- 230² Total area
- 4 rooms
- 1 bathroom
- 125.2² Total area
- 6 rooms
- 2 bathrooms
- 131.2² Total area
- 3 rooms
- 1 bathroom
- 124.3² Total area
It is customary to measure timber and rounded building materials in cubic meters for transportation and sales transactions. Let's look at ways to calculate the cubic capacity of a log and determine the number of pieces per cubic meter. Let's get acquainted with the sources where you can find ready-made accurate data, and understand the unjustification of using online calculators. After reading the article, it will be easier to create an estimate for building a house yourself.
essential oils
From the wood of camphor laurel (Cinnamomun camphora) cultivated in the Caucasus, camphor oil is obtained by steam distillation (oil yield 4%), which is used to prepare camphor. Fir oil is extracted from the needles and cones of different types of fir, which is a transparent, colorless aromatic liquid that quickly evaporates in air. Siberian fir needles contain from 0.63 to 3%, and Caucasian fir needles contain 0.2% fir oil. Fir oil is used in pharmaceutical production, perfumery and for the preparation of varnishes. Volatile essential oils of coniferous species of pine, spruce, and western thuja have phytoncidal properties, i.e., the ability to kill microbes in the air or water.
Lactoresins are milky juices of some plants, close to resins. These include rubber and gutta-percha. Rubber is extracted from the bark of the Hevea brasiliensis tree and is an amorphous mass from yellow to dark color, soluble in carbon disulfide, chloroform, ether and turpentine. Gutta-percha is obtained from some tropical tree species (for example, Isonandra gutta Hook, etc.). In the USSR (in the Crimea, in the Caucasus), eucommia is acclimatized, the leaves and roots of which contain 4-6% gutta-percha. Of the domestic species, gutta-percha is contained in the root bark (up to 7%) of Euonymus warty and European. Purified gutta-percha is a brown solid mass, easily soluble in carbon disulfide, chloroform and turpentine. It is used to make cliches for drawings, insulation of electrical cables, etc.
Dyes can be found both in wood and in bark, leaves and roots; Wood contains dyes of red, yellow, blue and brown. Of the species growing in our country, for dyeing fabrics and yarn yellow, the local population in the Caucasus uses maclura, mulberry, mackerel, hornbeam, sumac and hop hornbeam wood, for red dyeing - dry buckthorn bark, for brown - mackerel wood, peel walnut, etc.
Calculation of tree cubes
A tree is a perennial plant with a woody, erect trunk.
The volume of trees is calculated using a simple formula:
V = 3.1415 * d 2 / 4 * l * N
, Where
N is the number of trees; V is the total volume of trees; d—diameter of trees; l is the length of the trees.
You can quickly perform this simple mathematical operation using our online program. To do this, enter the initial value in the appropriate field and click the button.
This page presents the simplest online calculator for calculating tree cubes. With this calculator, in one click you can calculate the volume of wood in m3, if you know their number, length and diameter.
basic chemical reactions of wood of industrial importance
The interaction of wood with acidic salts of sulfurous acid and alkalis occurs in the processes of producing technical cellulose - the main semi-finished product in pulp and paper production. Methods for producing sulfite and sulfate cellulose are described above. Pulp production waste is used as raw material for chemical recycling. To make some types of paper, not only cellulose can be used, but also other organic substances contained in wood. In this case, the wood is subjected only to mechanical processing, which results in wood pulp. When wood is abraded (defibrated) pressed against the abrasive surface of a rapidly rotating stone, in the presence of water, a white wood pulp is formed, which is used to prepare papers that accept ink well when printed, but are characterized by low strength. If the wood is steamed before abrasion, it produces a brown wood pulp used to make strong wrapping paper and certain types of cardboard.
The interaction of acids with wood leads to the formation of the simplest sugars from polysaccharides and is used as the main reaction in hydrolysis production. At modern hydrolysis enterprises, which include a whole complex of chemical production, all the components of wood raw materials are used most fully and rationally. The raw materials for hydrolysis production are waste from sawmilling and wood processing. Hydrolysis of wood can be carried out in two ways: 1) with dilute mineral acids at high temperatures (under pressure) and 2) with concentrated acids at normal temperatures (without pressure). The first method has found the most widespread use. Raw materials in the form of sawdust or wood chips enter the hydrolysis apparatus - a vertical cylinder with a cone-shaped upper and lower part. Together with the wood, cooking acid, which is a 5% aqueous solution of sulfuric acid, is supplied to the hydrolyser. The temperature rises to 140-160° and saccharification (hydrolysis) of hemicelluloses occurs. Then the hydrolysis of cellulose begins with the continuous flow of cooking acid into the apparatus, heated to 185°, and the simultaneous selection of the hydrolyzate - an aqueous solution of simple sugars. The pressure in the apparatus during hydrolysis rises to 15 atm. At the end of cooking, instead of acid, hot water is supplied to wash the insoluble residue - hydrolytic lignin.
When the hydrolyzate is cooled, vapors are formed, from the condensate of which a number of products are obtained. The most valuable of them is a colorless oily liquid with the smell of burnt bread - furfural, which is used in the production of plastics, synthetic fibers such as nylon, resins, for the purification of lubricating oils, the manufacture of medicines (furacilin, etc.), dyes, and weed control products. , mushrooms and insects and for other purposes. Furfural can be obtained as the main product from the hydrolysis of pentosan-rich hardwood (birch, aspen) and agricultural plant waste.
The hydrolyzate (wort) neutralized with lime milk enters the fermentation department. There, under the action of distiller's yeast enzymes, the simple sugars contained in the wort - hexoses (glucose and sugars from hexosanes) - are fermented and form ethyl alcohol and carbon dioxide. The carbon dioxide released during fermentation is captured and used to produce liquid carbon dioxide and dry ice. Ethyl alcohol is used in the production of synthetic rubber and in many other industries. However, it is now recognized as more economically feasible to satisfy the basic need for alcohol with synthetic alcohol obtained from ethylene petroleum gases.
The residues after distillation of alcohol (stillage) contain undecomposed pentoses, which are used for growing feed yeast, rich in vitamins and protein. Their introduction into the diet of birds and animals sharply reduces mortality, increases the rate of meat growth, etc. Considering the importance of feed yeast for increasing the productivity of livestock, poultry and fish farming, you can refuse to produce alcohol and use the entire hydrolyzate to grow yeast. In this case, it is necessary to use such cultures of yeast and yeast-like fungi that are capable of assimilating not only pentoses, but also hexoses.
Thermal decomposition (pyrolysis) occurs when wood is heated without access to air (dry distillation) or with limited air supply (gasification). During dry distillation of wood, water is first removed from the outside using heat (at temperatures up to 120-150°) and the wood is partially decomposed with the release of carbon dioxide, carbon monoxide and acetic acid vapor (at temperatures 150-270°). Then, at a temperature of 275°, the main decomposition reactions of the substances that make up the wood occur. This phase of the process occurs with rapid release of heat. The last stage of pyrolysis with additional external heating occurs at a temperature of 300-400° and consists of calcining the coal to remove the residue - volatile substances. As a result of dry distillation, solid (coal), liquid (liquid) and gaseous products are formed.
During the thermal decomposition of pine, spruce, birch and beech wood under atmospheric pressure, a final temperature of 400 ° C and a heating duration of 8 hours, approximately 32-38% coal, 45-50% liquid and 15-20% gases (including losses) are obtained. The greatest importance now is coal, which is free from mineral impurities (sulfur).
Coal is used in metallurgy as fuel for the smelting of non-ferrous metals, for the production of carbon disulfide used for the production of viscose fiber, for the production of activated carbon, electrodes, etc. Charcoal is obtained as the main product in charcoal burning. Liquid, or aqueous distillate, is an aqueous solution of wood decomposition products. From the resin formed after settling of the liquid, phenols are obtained for the production of plastics, gasoline antioxidant, flotation oils for ore beneficiation and other products. The liquid is also used to produce methyl alcohol and acetic acid. The largest number of these products are obtained from hardwood.
In connection with the development of methods for producing synthetic methyl alcohol and acetic acid, the importance of these products of dry distillation of wood has decreased. The gases generated during the pyrolysis of wood are used as fuel to heat retorts (dry distillation apparatus). Dry distillation of bark produces more resin, coal and gases, but less acetic acid and methyl alcohol. The yield of the main products of dry distillation of wood and bark as a percentage of the mass of raw materials in an absolutely dry state is indicated in the table. 10. Here are the data for pine and birch. The existing proposals for combining wood hydrolysis with dry distillation are interesting. Pyrolysis of birch wood, pre-impregnated with 1% sulfuric acid, allows one to obtain a significant amount of furfural.
yield of main products during dry distillation
| Products | Output, %, from | |||
| pine trees | birch trees | |||
| wood | bark | wood | bark | |
| Coal | 37,90 | 42,50 | 33,0 | 37,40 |
| Gases | 18,20 | 19,80 | 15.3 | 18,50- |
| Acetic acid | 3,10 | 0,85 | 6,9 | 2,55 |
| Methyl alcohol | 0,85 | 0,31 | 1,6 | 0,69 |
| Resin | 7,00 | 8,40 | 6,3 | 14,90 |
Gasification of wood in energy-chemical installations, which make it possible to produce generator gas and capture pyrolysis products, can serve as one of the methods for recycling wood waste. The oxidation of wood during combustion is important if it is used as fuel. The quality of wood as fuel is assessed by its calorific value. Mass calorific value is the amount of heat that is released during the complete combustion of a unit of mass - 1 kg of wood.
where C, H and O are the content of carbon, hydrogen and oxygen,%; W is the relative humidity of wood. This formula gives only approximate values, deviating from the actual ones by 5-10%. The exact calorific value is determined in the laboratory in calorimeters using standard methods. The mass calorific value practically does not depend on the type of wood; this is explained by the same chemical composition of wood of different species. Thus, according to available data, the mass calorific value of absolutely dry wood ranges from 4700 to 5100 kcal. The calorific value of absolutely dry birch bark is higher than that of wood by 17%, and that of alder by 12%.
In practice, firewood is assessed not by mass (weight), but by volume; in this case, the volumetric or specific calorific value of wood is important, i.e. the amount of heat produced by the combustion of a unit volume of wood. The specific heating value can be obtained by multiplying the mass heating value by the density of the wood.
The calorific value depends to a large extent on humidity: as the moisture content of wood increases, it decreases. The highest temperature that can be achieved under ideal combustion conditions (heat-producing capacity of wood) can also be calculated theoretically. In laboratories it is determined by pyrometers. So, for absolutely dry beech wood, the combustion temperature is 1720°. However, practically, due to losses in the firebox, such a temperature cannot be achieved; the actual combustion temperature of wood can be taken equal to 1000-1100°. Currently, the importance of wood as a fuel is decreasing due to the widespread use of high-calorie liquid and gaseous fuels.
Soot
Soot (soot, tar) are particles consisting primarily of carbon. In the presence of temperature and oxygen, they burn. If this is not the case, then they stick together into large pieces and settle on the walls of the stove and chimney, but they can also fly away into the chimney. Not only does this require cleaning the oven, it can catch fire, it also reduces its efficiency, because... a layer is formed that prevents heat absorption by the oven walls. On the issue of insufficient knowledge of the combustion process: in 1996, Robert Curl was awarded the Nobel Prize for the discovery of fullerenes in soot.